This article has been cited byother articles in PMC. Calculate the heat of solution AH goln Specific heat of the solution SH0961 calgC and molar mass of MgSO4 MW120366 gmol.

Find The Heat Of Dissolving Delta H Dissolution Youtube
266 20 gcm 3 s ΔHmelt molar enthalpy of melting.

. The enthalpies of formation of synthetic MgSO44H2O starkeyite and MgSO43H2O were obtained by solution calorimetry at T29815 K. It has a role as an anticonvulsant a cardiovascular drug a calcium channel blocker an anaesthetic a tocolytic agent an anti-arrhythmia drug an analgesic and a. 7H2Os -3673623506-183748479 -5511108296 -551kJmol-1.
Calculate the molar heat of solution for magnesium sulfate Jmol. Up to 24 cash back q m xcx ΔT Equation 1. For this we use the equation QmcΔt where Q heat energy m mass cspecific heat capacity and Δt the change in temperature for the process.
Magnesium sulfate is usually encountered in the form of a hydrate MgSO. MgO 4 S Structural formula as text. Most students and the teacher immediately calculated heat of reaction 100g418Jgc525c2190J but calculating the heat gained by the calorimeter first then heat of solution and finally heat of reaction.
Copy Sheet of paper on top of another sheet. The true standard enthalpy change of the hydration of magnesium sulphate is -1040 kJ mol-1. Magnesium sulfate heptahydrate H14MgO11S CID 24843 - structure chemical names physical and chemical properties classification patents literature biological.
ΔG298 standard molar Gibbs energy of reaction at 298 К. 500 g NaOH x 1 mol 40g 0125 mol. Where mis the total mass of the solutionsolute plus solvent cis the specific heat of the solution and ΔTis the observed temperature change.
Molar mass of NaOH 2299 1600 101 40. Calculate the molar heat of solution for magnesium sulfate. Colorless rhombic crystals Empirical formula Hills system for organic substances.
Molar mass of MgSO 4 Magnesium Sulfate is 1203676 gmol. View the full answer. We review their content and use your feedback to keep the quality high.
Mass percentage of the elements in the composition. Ion-interaction Pitzer equations are fitted to these data with additional guidance for Csub. Standard molar enthalpy heat of formation.
ΔH298 Standard molar enthalpy of reaction at 298 К. Q500g4084kJk0220K 449 Kjmol. Using q p C p T C p T 49324 JK x 375 - 25K 61655 J.
1165175 kJ 0125 mol 93214 kJmol. Articleosti_5954589 title Heat capacity and other thermodynamic properties of aqueous magnesium sulfate to 473 K author Phutela R C and Pitzer K S abstractNote Measurements are reported for the heat capacity of aqueous MgSOsub 4 from 348 to 473 K. The enthalpy change of the hydration of magnesium sulphateHr Hsoln MgSO4s- Hsoln MgSO4.
We are given three pieces of information in this formula mc and Δt so we can find the fourth Q which equals molar heat of the solution of KCN. 50 salt added na. Include the correction due to the heat capacity of the calorimeter.
The specific heat of the solution is generally assumed to. 1 mass of magnesium sulfate 42 g Molar mass of magnesium sulfate 120 gmol Number of moles of magnesium sulfate mass molar mass 42 g 120 gmol 035 moles Given Volume of water 750 mL Density of water 1 gmL Mass of water. Give your answer for q values as it.
Copy Sheet of paper on top of another sheet. The molar heat of solution of NaOH is -4451 kJmol. Magnesium sulfate or magnesium sulphate in English-speaking countries other than the US is a chemical compound a salt with the formula MgSO 4 consisting of magnesium cations Mg 2 2019 by mass and sulfate anions SO 2 4It is a white crystalline solid soluble in water but not in ethanol.
Heat Lost 548625 J 61655 J 11 65175 J 1165175 kJ. Magnesium sulfate is a magnesium salt having sulfate as the counterion. In a certain experiment 500 g of NaOH is completely dissolved in 1000 L of 200C water in a foam cup calorimeter.
The resulting enthalpies of formation from the elements are starkeyite2498711 kJmol1and MgSO43H2O2210313 kJmol1. Assuming no heat loss calculate the final temperature of the water. The temperature of the solution in the calorimeter increased from 280 C to 380C.
Product name Magnesium Sulfate MgSO4 Solution 100 mM Product No B1003 Recommended use of the chemical and restrictions on use Recommended use This product is for research and development only Uses advised against No information available Details of the supplier of the safety data sheet Supplier Address New England BioLabs 240 County Road. Magnesium sulphate Group of substances. Calculate the molar heat of solution for magnesium sulfate Include the from AP CHEM 270021 at La Sierra University.
In a calorimetry experiment 150 g of magnesium sulfate MgSO was dissolved in 2000 g of water. Ionic Solute Enthalpy of Solution Hsoln kJmol BaCl2 anhydrous -867 BaCl2 2H2O 2058 MgSO47H2O 1611 NH4NO3 2644 Na2S2O3 5H2O 4740 Calculate the energy change if 365 g of magnesium sulfate heptahydrate is dissolved in water to make a 1500 mL solution. Q mass heat capacity Temp Temp 273-21261 q 50018 4184 61 q1276579403 Joules DeltaT 1173 61 1173 71553 Heat lost qTotal Heat 71553 1276579403 1992109403 J to KJ 1992109403.
I was just wondering if I did that correctly. 28 rows Temperature of MgSO 4 7 H 2 O solution ºC Temperature of MgSO 4 solution ºC 00.
Calculate The Molar Enthalpy Of Solution
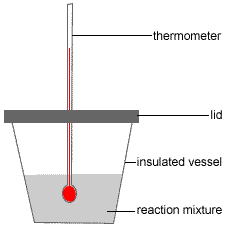
0 Comments